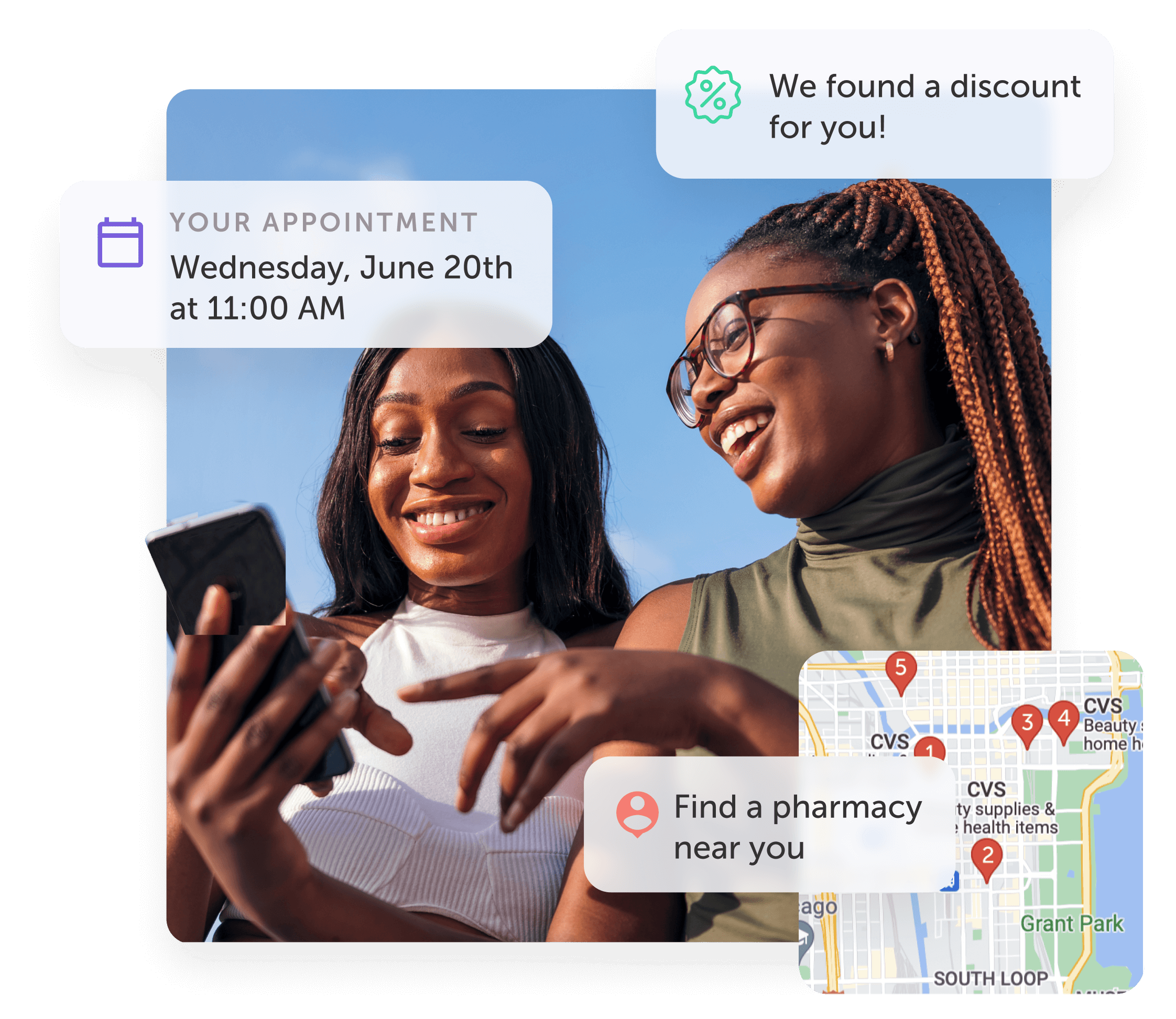
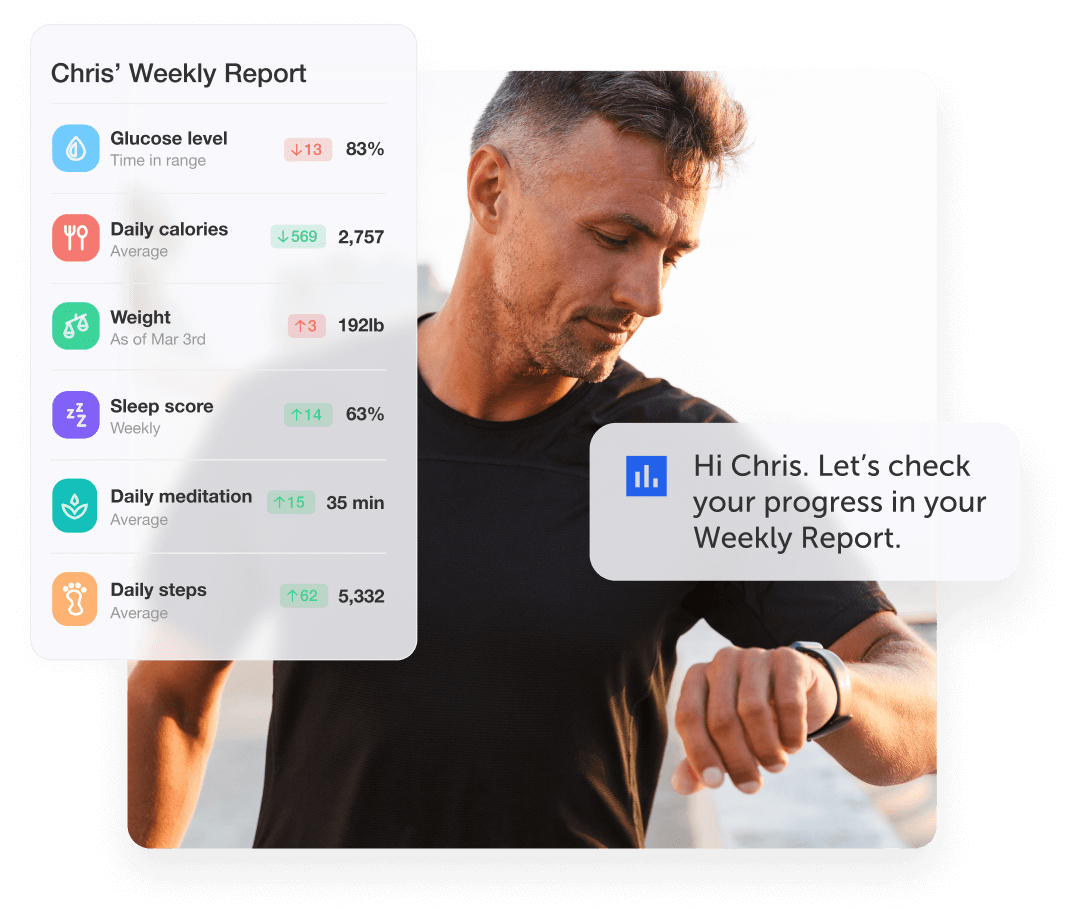
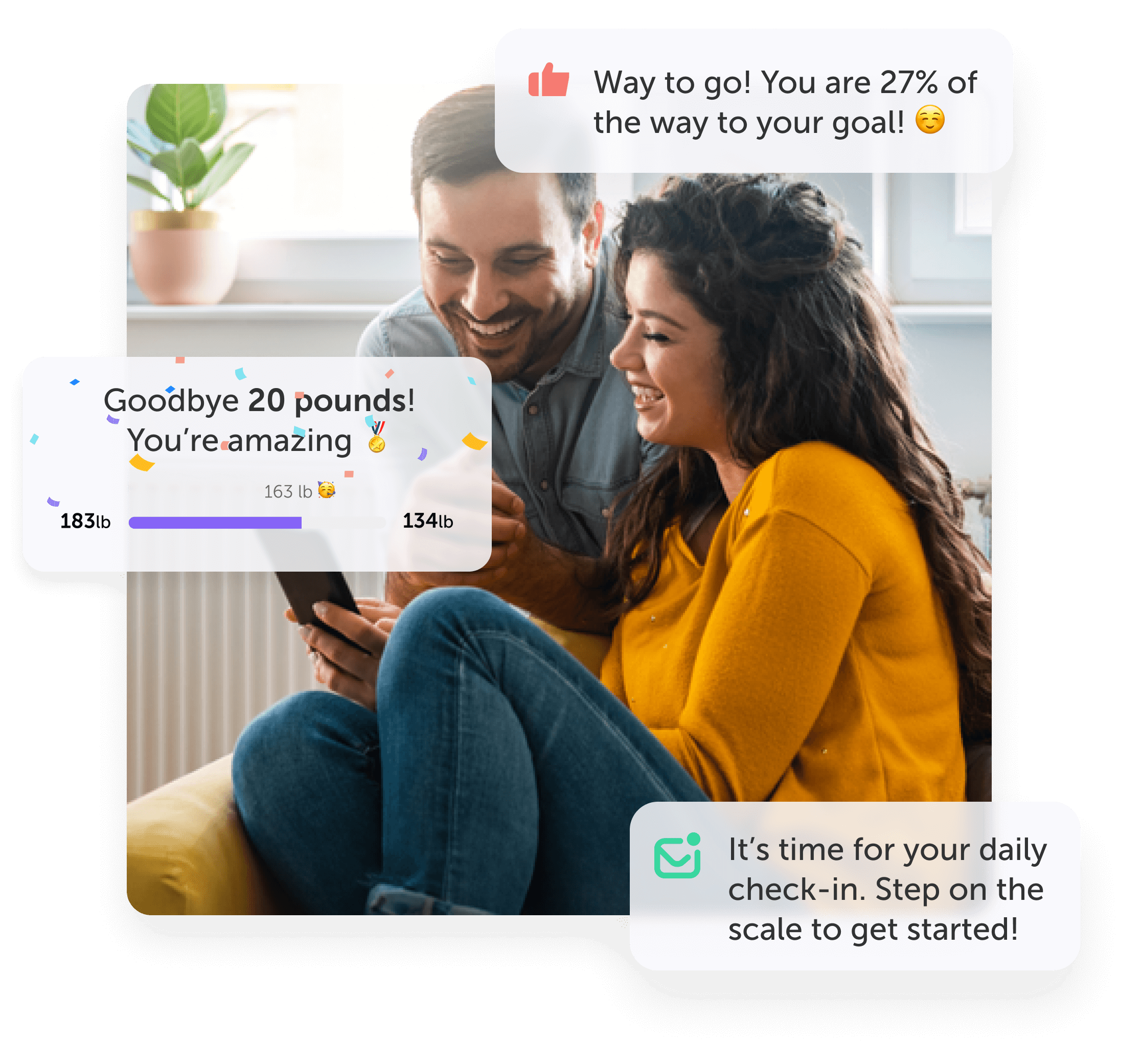
Making healthcare delightful, accessible, and personalized
We are trusted by leading enterprises, government, and consumers everywhere
Upgrading care delivery by turning transactions into relationships
Our platform powers seamless user experiences:
We deliver leading-edge technology infrastructure that's extensible, scalable, compliant and secure
We enable delightful healthcare experiences that improve users health and wellbeing, and promote joy and satisfaction
We help integrate fragmented healthcare services seamlessly, efficiently, and cost-effectively
Improving healthcare engagement
Our proprietary AI helps users reach better health:
Safe and valuable education
Personalized curated guidance to care solutions
Motivation and engagement
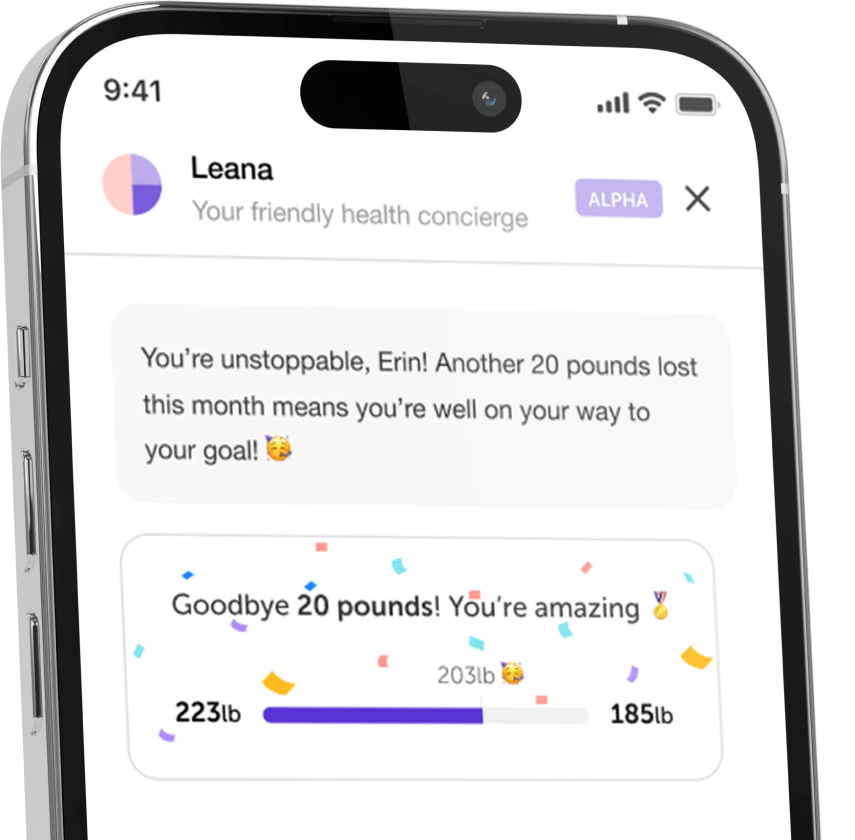
Let's make healthcare personal again, together!
Award winning experience
Safe, secure, trusted, and compliant
 SOC 2 TYPE II CERTIFIED
SOC 2 TYPE II CERTIFIED
Select On/Go apps
We've validated our platform + AI with many millions of users through our top-rated On/Go apps
Explore our award-winning apps